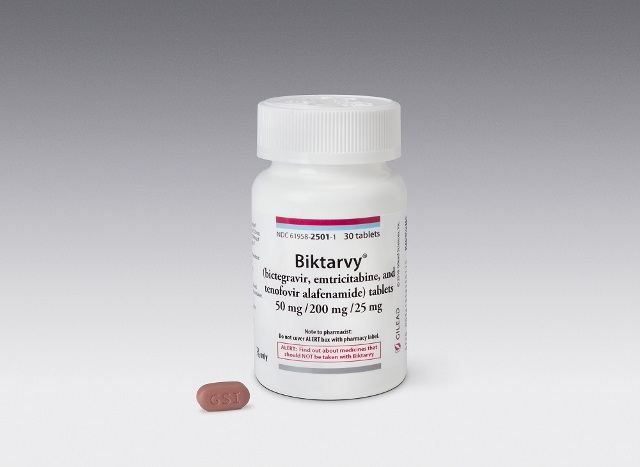
In an attempt to boost Guyana’s efforts to eliminate the Human Immunodeficiency Virus (HIV), the Ministry of Health partnered with American biopharmaceutical company GILEAD Sciences Inc., to distribute a new HIV drug called Biktarvy, which is an improved and more developed drug to treat adults living with HIV.
The new drug will add to the ministry’s already robust programme, which includes the roll out of comprehensive pre-exposure prophylaxis (PrEP) and self-testing for patients, among other initiatives.

“We have had a very robust programme over the years in HIV, and right now over the last two years or so we’re even pushing the boundary with what we think are the newer innovations in HIV prevention and treatment,” health minister, Dr. Frank Anthony, M.P. said Thursday evening at the launch at the Marriott Hotel, Georgetown.
He said the new drug comes at an opportune time as the ministry is currently reviewing its HIV treatment protocols.

The drug is a complete, one-pill, once per day prescription medicine. While other HIV drugs have shown resistance in some patients, there has been no recorded case of resistance thus far with this new treatment.
Meanwhile, Infectious Diseases Specialist (US/Guyana), Dr. Moti Ramgopal said the World Health Organisation (WHO) has now set new targets for countries to achieve the ‘95/95/95 goal’ by 2030, that is; identifying persons with HIV, treating those persons and ensuring they are virally suppressed.

To achieve this goal, he said there must be less than 100, 000 new cases per year.
“When you look at the last 20 years, this has a lot to do with medication improvements, access to medications, testing, access to testing and just really pushing through the hard around the world,” Dr. Ramgopal said.
The specialist also noted the need to bridge the gaps in testing and treating patients living with the infection.

